Safe air travel, masks, and the limitations of PPE
Guidance from the Health & Safety Executive is such that PPE facemasks should not be worn in oxygen reduced or hypobaric atmospheres, such as aircraft, and this is legally binding on all aircraft landing in and crossing British airspace.[i]
Virustatic has prepared this report to enable decision and policy makers to better understand viral transmission routes and protective measures on aircraft and recommends consideration of the Biological Cloth Face Covering technology as part of scientifically advanced safety initiatives to reinstate international air travel.
Introduction. Aircraft cabins as potential transmission vectors.
COVID-19, a disease caused by the SARS-CoV-2 virus, was first reported to the WHO Country Office in China on 31st December 2019 and the virus has been spreading from country to country, following modern travel routes.
Social distancing and hygiene measures are methods currently mandated to prevent transmission of SARS-CoV-2 and other respiratory viruses. There has been no requirement by aviation authorities such as the European Union Aviation Safety Agency, the US Federal Aviation Administration or the UN-agency ICAO to make social distancing mandatory on-board.
Airlines have introduced other safety measures, such as mandatory face masks for passengers and crew, enhanced cabin cleaning and changing boarding and in-flight processes to reduce interpersonal contact.
On 31 May 2020, the French Government made the wearing of a surgical mask compulsory on board flights (decree n° 2020-663 on 31st May 2020). Other airlines and ferry carriers have implemented similar regulations to wear surgical masks – de facto if not de jure – and passengers wearing face coverings that are not in the ‘duck bill’ style have been asked to remove them in exchange for a single-use surgical mask.
Figure 1: Example of a surgical mask on board a KLM flight
The Air France website states that, on board, “the quality standard of the air ventilation equipment used renders the risk of viruses and microbes spreading impossible.”[ii] The notion that virus and microbe transmission is ‘impossible’ on flights is both incorrect but also contradictory to their mandatory use of surgical facemasks, which have been shown to be poor-performing when it comes to viruses in aerosols.
There is evidenced high transmissibility of COVID-19 in confined spaces and the spatial layout of aircraft, the physical characteristics of in-flight environments, ventilation flows and proximity of passengers all play a role in the spread of COVID-19.
To remain trading, airlines must encourage trust and increase the perception of flight safety so that they can continue to operate. Genuinely effective changes and improvements to onboard safety from infection need to consider many different disciplines: virology, microbiology, epidemiology, medicine, engineering, and physics.
A separate consideration is the environmental impact of all air passengers using single-use surgical masks. A report in Environmental Science & Technology estimates that during the pandemic we are using 129 billion disposable face masks per month.[iii] Mandatory single-use PPE on-board will add multiple millions to this figure and affect sustainability objectives.
Pathogen exchange pathways on aircraft
Airborne particles exhaled by an infected person can travel distances of metres or tens of metres in the air and carry viral content.[iv] Despite this, no countries, authorities or airline carriers have considered airborne spread of COVID-19 in their regulations to prevent infection transmission onboard.
Viruses can be transmitted in aircraft cabins through the following three modes of action:
Close contact due to proximity of passengers and high occupant density on board
The prevalence of surfaces or fomites.
This is when a shedding person (who may be asymptomatic even if in receipt of a negative COVID-19 test within the last 72 hours) touches a surface or when large virus-containing droplets have landed on the surface. Fomites include objects, materials and surfaces such as trays, seats, arm rests, overhead cabins, handles, inflight entertainment screens and controllers, seatbelts, toilet doors etc. on which virus-laden droplets land and can remain stable for days).Airborne viral exchange and transfer of viruses in aerosol due to air flows and viral plumes, regardless of HEPA filters.
Pathogens can become airborne when droplets are generated during speech, coughing, sneezing, vomiting, or via the atomisation of faeces. Droplets smaller than 5 µm and desiccated droplet nuclei are known as aerosol, which can remain airborne for several hours ([v], [vi], [vii]) and are so small that transport by air current affects them more than gravitation.
Current regulations regarding mask wear and social distancing rules are based on estimations of risk of droplet transmission in relation to isolated large droplet emission only, and not droplets at the smaller end of the droplet size continuum which may be embedded in a cloud of exhaled air which are invisible to the naked eye.
It is therefore extremely important that authorities and airlines acknowledge the reality that the virus can survive and spreads through air and recommend that adequate control measures be implemented to prevent further spread of the SARS-CoV-2 virus.[viii]
Airborne transmission of SARS-CoV-2 and other respiratory viruses
Van Doremalen et al, analysed SARS-CoV-2 across 10 experimental conditions in five environments and showed that the virus is stable in air for at least 3 hours,[ix] with others suggesting it may be stable for up to 16 hours.[x]
Airborne transmission route of the virus by respirable particles (< 10 µm) is important when it comes to air travel because they are long range and subject to the airflows of their particular indoor space.
Smaller airborne droplets laden with SARS-CoV-2 may spread up to 8 metres in exhaled air from infected individuals, even without background ventilation or airflow.[xi]
Multiple analyses, in indoor settings other than in air cabins, support probable airborne transmission of COVID-19 ([xii], [xiii], [xiv], [xv]) in situations when close contact did not occur. Indeed, one study showed that specific patterns of infection can be attributed to the flow lines from ventilation,[xvi] providing further evidence that airborne viruses can be carried in air flows.
Airborne transmission of coronaviruses has been suspected since the last outbreak of SARS-CoV and the global spread by air travellers and in-flight spread of SARS-CoV was known and documented. However, even though airborne transmission of COVID-19 is now accepted, the disease still mostly prevented against as if spread by large, aerosolised droplets or by direct and indirect contact, (i.e. social distancing and surgical masks). However, airborne or small droplet transmission better explains the distribution of SARS cases that occurred on commercial airlines. ([xvii], [xviii])
Reviewing SARS-CoV is relevant, as van Doremalen et al. found that the stability of SARS-CoV-2 was similar to that of SARS-CoV-1 under experimental circumstances. In the absence of in-flight studies of SARS-CoV-2 transmission, we should examine historic studies of SARS-CoV and extrapolate.
Figure 2. Schematic diagram of SARS outbreak aboard Hong Kong to Beijing flight. From Olsen SJ, Chang HL, Cheung TY. Transmission of severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349:2416–2422
Evidence suggests that transmission of SARS-CoV during the Amoy Gardens outbreak in Hong Kong was a result of airborne spread via a viral plume.[xix] One 3-hour flight carrying 120 passengers travelling from Hong Kong to Beijing on March 15, 2003 began a superspreading event accounting for 22 of the 37 people who contracted SARS after air travel.([xx], [xxi]) The number of secondary cases from that flight remains unknown, but more than 300 people were thought to be affected.[xxii] Figure 1 shows the distribution pattern of the SARS-CoV outbreak on this flight. The distribution pattern of SARS-CoV transmission on the flight emphasised the need to consider airborne transmission patterns aboard commercial aircraft[xxiii] and, until it can be ruled out, we should assume that SARS-CoV-2 may spread in the same way.
This pattern in Figure 2 is important because it did not follow the typical example of in-flight transmission of pathogens—i.e. sitting within two rows of the index passenger. The duration of the Hong Kong to Beijing flight was just three hours long and affected passengers were seated seven rows in front and five rows behind the index passenger. Explanations for this outbreak distribution must include airborne transmission rather than direct contact spread; a malfunctioning cabin filtration system; and passengers infected before or after the flight.[xxiv]
Transmission prevention with use of face coverings and facemasks; the problem with mandated surgical masks
Figure 3: Example of an incorrectly worn surgical mask on flight, behind a Virustatic BCFC wearer
Wearing a mask or face covering should reduce the potential for transmission through air flow and cabin ventilation mediated pathways.
However, surgical masks are very often incorrectly handled and worn, as seen in Figure 3. The material in these surgical masks is hydrophobic and they are designed to provide a barrier so that liquid particulates or splatter, such as mucus and blood, are caught on the surface of the mask. The virus-laden droplets remain live on the surface of the mask so they are another fomite which can go on to cause infection when the mask is touched, removed – for eating and drinking, which is permitted on board – and disposed.[xxv]
Research on facemasks and coverings from the University of Edinburgh showed that surgical masks generate several intense leakage jets which may present major hazards.[xxvi] Ejected airflows showed that these surgical masks redirect the jets (from coughs, sneezes and breathing) downwards and sideways. The study also noted the comparatively high strength and diffusivity of the virus-laden jets coming out of surgical masks compared to the other jet types and this would be hazardous when on aircraft, given the pattern of passengers’ seats.
The study highlighted another potentially dangerous leakage jet which is the backward jet from surgical masks. Air escapes from the side of the mask and it is projected backwards at high speed, potentially resulting in a significant displacement. The backward jet is produced by a person breathing with a surgical mask. This jet was not produced by FFP1 and FFP2 masks. The study states that it is ‘particularly pronounced for surgical masks.’
It was observed in the same study that when wearing certified ‘face fit’ Personal Protective Equipment (PPE) masks, increased pressure caused by exhaling – and particularly coughing and sneezing – displaced the mask from the face leading to air leakage around its perimeter.[xxvii]
The directionality of these potentially virus-laden jets should be a main consideration for airlines, legislators and passengers in order to avoid a false sense of security that may arise when sitting to the side of, or behind, a person wearing a surgical mask.
Virustatic Biological Cloth Face Coverings.
The Virustatic BCFC is the result of over 10 years of research and development and it is designed for prolonged wear and handling. Safe air travel was in mind during its design and its efficacy is unaffected at high altitude, low oxygen and low relative humidity i.e. in-flight conditions. It is effective against the airborne transmission of viruses in airplane cabins. The material used is hydrophilic and highly breathable, and so does not produce the expulsion of strong leakage jets which circumvent the covering. The snood style also mitigates the sideways and upwards ingress and sideways, backwards and downwards egress jets of virus-laden particles.
The material is coated in patented Viruferrin ™ which contains the glycoprotein lactoferrin, which adds additional protection as it captures airborne pathogens such as influenza, coronaviruses and adenoviruses. Tests showed that it was effective at blocking and capturing up to 99% of influenza (H1N1) and SARS-CoV-2 on its surface. Viruferrin ™ binds viruses on the surface of the material so they cannot be inhaled or retransmitted by touch when adjusting or removing the snood. It will also prevent virus-laden particles that come into contact with the material from becoming airborne
The material’s high breathability sustains a low pressure drop and remains fitted to the face even when sneezing and coughing. This unique action offers protection in both directions. It does not require fit-testing.
The smallest airborne particles are filtered through electrostatic attraction and diffusion filtration while the largest droplet particles are captured through impaction and interception and then desiccate on the surface of the masks, lowering the pH of the virus particle, thereby inactivating it. Early evidence shows a direct binding action of the lactoferrin to the spike protein.
The Viruferrin™ protein coating’s antimicrobial peptides have a cationic property which disrupts and inactivates the influenza virus and the SARS-CoV-2 virus contained in the smallest mucus aerosol droplets.
Why is infection more likely on aircraft than other indoor spaces?
1) Aircraft Ventilation and filtration
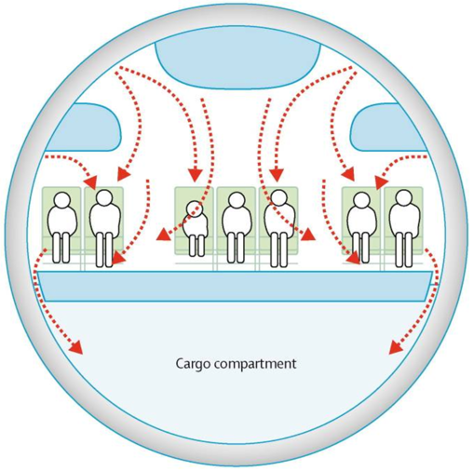
Figure 4. Air circulation pattern in typical airline passenger cabin. Arrows show air currents. WHO: Tuberculosis and air travel: guidelines for prevention and control. WHO/TB98.256. World Health Organization; Geneva, Switzerland: 1998.
Natural ventilation can be one of the effective environmental measures to reduce the risk of spread of infections.[xxviii] However, a higher than normal viral load is likely to be experienced by aircraft passengers and cabin crew as a consequence of the much lower rate of outside air ventilation per person in modern aircraft than for any other ground level or transport public space.([xxix], [xxx]) To illustrate, other public spaces routinely provide 7 to 14 L/s of outside air per person, while aircraft supply those flying with only an average of 4.8 L/s.[xxxi] It seems inevitable that this reduced rate of provision of outside air in modern aircraft increases viral load in the cabin.
HEPA filters used on aircraft are rated to remove at least 99.97% of particles at 0.3 µm in size, representing the most penetrating particle size.[xxxii] Most viruses, including CoVs, range from 0.004 to 1.0 µm[xxxiii] with a size of 0.09 to 0.12 µm for influenza virus [xxxiv] and an individual SARS coronavirus ranges from 0.075 to 0.160 µm in diameter, and is a spherical virion.
However, viruses are rarely observed as individual particles, but instead are expelled from the body already combined with water, proteins, salts, and other components as large droplets and aerosols. Thus far, SARS-CoV-2 has been observed in aerosolised particles in a spectrum of sizes, including 0.25 to 0.5 µm[xxxv], showing that even high efficiency filtration techniques, such as HEPA filters, could be penetrated by particles at the smaller end of the size continuum.
Additionally, it has been found that gaps at the edges of filters in a hospital setting has been a contributing factor of the failure of filtering systems to eliminate pathogens from the shared air environment.[xxxvi] This would also be a concern for inflight ventilation systems.
Viruses can not only be transported by ambient air currents (caused by someone standing up, a steward passing, or the WC door opening) but also by ventilation system flows (see Figure 3). This means clouds of droplets and aerosols can be displaced into the neighbouring environment.
Proper filter installation and maintenance can help reduce the risk of airborne transmission, but it is important to understand that filters should not be assumed to eliminate airborne transmission risk.
Viral shedding (higher with coughing/sneezing) and factors related to airflow in indoor environments, such as ventilation, may increase droplet spread. Nishiura et al report that the odds of transmission in an enclosed environment were 18.7-fold higher than in an outdoor environment.[xxxvii]
2) Humidity
Lower relative humidity (RH) has been proven both to increase human susceptibility to viruses and for viruses to retain maximal infectivity. Maintaining a RH between 40% and 60% may help to limit the spread and survival of SARS-CoV-2 within the aircraft.
However typical RH in aircraft cabins is lower than 20%. The RH for flights over an hour is below 10% for most of the journey, often dropping to less than 5% on longer flights.
Humidity plays a role in the survival of membrane-bound viruses, such as SARS-CoV-2.([xxxviii], [xxxix], [xl]) Previous research has found that, at typical indoor temperatures, relative humidity (RH) above 40% is detrimental to the survival of many viruses, including CoVs in general ([xli], [xlii], [xliii]) and higher indoor RH has been shown to reduce infectious influenza virus in simulated coughs.37
Based upon studies of other viruses, including CoVs, higher RH also decreases airborne dispersal by maintaining larger droplets that contain viral particles, thus causing them to deposit onto surfaces more quickly. ([xliv], [xlv], [xlvi])
Furthermore, changes in humidity can impact how susceptible an individual is to infection by viral particles and also lowers the innate resistance to infection.[xlvii] It also affects how far into the respiratory tract viral particles are likely to deposit.[xlviii] Decreased RH has been demonstrated to decrease mucociliary clearance of invading pathogens and weaken the innate immune responses. ([xlix], [l], [li])
Low RH, such as the standard on aircraft, is optimum for both human susceptibility and the stability and infectivity of viruses.
3) Reduced oxygen levels
Although there is a paucity of evidence on the effects of air travel on oxygen saturation in general populations, the peripheral oxygen saturation (SpO2) and pulse rate of 84 passengers, aged 1-78 years, have been measured by pulse oximetry at ground level and altitude during air travel. There was a statistically significant reduction in oxygen saturation in all passengers travelling long haul and short haul flights. The mean SpO2 for all flights at ground level was 97% and at cruising altitude SpO2 was 93%. Fifty-four per cent of passengers had SpO2 values of 94% or less at cruising altitude. This is a level that would prompt physicians to administer supplemental oxygen in hospital patients.[lii]
This problem is further compounded by the empirical interpretation of complaints that FFP2 masks induce:
1. an increase of heart frequency;
2. the sensation of the shortness of breath; and
3. light-headedness and headaches.
These are symptoms of reduced oxygen levels, known as hypoxia. A study on healthy oral surgeons shows that wearing an FFP2 for an extended period of time induces a reduction in circulating O2 concentration.[liii]
Many test results indicate that an N95 mask induces discomfort in breathing, an increase in fatigue and a decrease in both mental and physical performance, accuracy.[liv] HSE guidance is such that PPE facemasks should not be worn in oxygen reduced or hypobaric atmospheres such as aircraft and this is legally binding on all aircraft landing in and crossing British airspace.[lv]
References:
[i] Executive S. A health risk assessment of working in hypoxic atmospheres Prepared by the Health and Safety Executive RR1137. Accessed: 22nd March, 2021: <https://www.hse.gov.uk/research/rrhtm/rr1137.htm>
[ii] https://www.airfrance.co.uk/GB/en/common/page_flottante/information/faq-coronavirus.htm
[iii] J.C. Prata, A. Silva, T.R. Walker, A.C. Duarte, T.A.P. Rocha-Santos. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol., 54 (2020), pp. 1-6, 10.1021/acs.est.0c02178
[iv] Morawska L, Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ Int. 2020;139:105730. doi:10.1016/j.envint.2020.105730
[v] Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2(3):143-154. doi:10.1080/15459620590918466
[vi] Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12(11):1657-1662. doi:10.3201/eid1211.060426
[vii] van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New England Journal of Medicine 2020;382(16):1564-67. doi: 10.1056/NEJMc2004973
[viii] Morawska L, Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ Int. 2020;139:105730. doi:10.1016/j.envint.2020.105730
[ix] van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New England Journal of Medicine 2020;382(16):1564-67. doi: 10.1056/NEJMc2004973
[x] Fears AC, Klimstra WB, Duprex P, et al. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv 2020:2020.04.13.20063784. doi: 10.1101/2020.04.13.20063784
[xi] Qureshi, Z., Jones N., Temple R., Larwood J., Greenhalgh,. Bourouiba L., What is the evidence to support the 2metre social distancing rule to reduce COVID-19 transmission? (2020)
[xii] Hijnen D, Marzano AV, Eyerich K, et al. SARS-CoV-2 Transmission from Presymptomatic Meeting Attendee, Germany. Emerging infectious diseases 2020;26(8) doi: 10.3201/eid2608.201235
[xiii] Döhla M, Wilbring G, Schulte B, et al. SARS-CoV-2 in environmental samples of quarantined households. MedRxiv 2020 doi: https://doi.org/10.1101/2020.05.28.20114041
[xiv] Park SY, Kim YM, Yi S, et al. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg Infect Dis 2020;26(8) doi: 10.3201/eid2608.201274 [published Online First: 2020/04/24]
[xv] Xu P, Qian H, Miao T, et al. Transmission routes of Covid-19 virus in the Diamond Princess Cruise ship. medRxiv 2020:2020.04.09.20059113. doi: 10.1101/2020.04.09.20059113
[xvi] Li Y, Qian H, Hang J, et al. Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. medRxiv 2020:2020.04.16.20067728. doi: 10.1101/2020.04.16.2006772
[xvii] S.J. Olsen, H.-L. Chang, T.Y.-Y. Cheung, A.F.-Y. Tang, T.L. Fisk, S.P.-L. Ooi, et al. Transmission of the severe acute respiratory syndrome on aircraft N. Engl. J. Med., 349 (2003), pp. 2416-2422
[xviii] WHO. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). WHO/CDS/CSR/GAR/ 2003.11. World Health Organization; Geneva: 2003. http://www.who.int/csr/sars/en/WHOconsensus.pdf (accessed 23 August, 2020)
[xix] Yu IT, Li Y, Wong TW. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739.
[xx] S.J. Olsen, H.-L. Chang, T.Y.-Y. Cheung, A.F.-Y. Tang, T.L. Fisk, S.P.-L. Ooi, et al. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med., 349 (2003), pp. 2416-2422
[xxi] Wilder-Smith A, Leong H, Villacian J. In-flight transmission of severe acute respiratory syndrome (SARS): a case report. J Travel Med. 2003;10:299–300.
[xxii] Lakshmanan I. Air China flight 112: tracking the genesis of a plague. Boston Globe. May 18, 2003:A1
[xxiii] Mangili A, Gendreau MA. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365(9463):989-996. doi:10.1016/S0140-6736(05)71089-8
[xxiv] S.J. Olsen, H.-L. Chang, T.Y.-Y. Cheung, A.F.-Y. Tang, T.L. Fisk, S.P.-L. Ooi, et al. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med., 349 (2003), pp. 2416-2422
[xxv] Chughtai, A.A., Stelzer-Braid, S., Rawlinson, W. et al. Contamination by respiratory viruses on outer surface of medical masks used by hospital healthcare workers. BMC Infect Dis 19, 491 (2019). https://doi.org/10.1186/s12879-019-4109-x
[xxvi] Viola, I. M., Peterson, B., Pisetta, G., Pavar, G., Akhtar, H., Menolascina, F., Mangano, E., Dunn, K., Gabl, R., Nila, A., Molinari, E., Cummins, C., Thompson, G., Lo, M., Denison, F., Digard, P., Malik, O., Dunn, M. J. G., & Mehendale, F. (2020). Face Coverings, Aerosol Dispersion and Mitigation of Virus Transmission Risk. arXiv.org.
[xxvii] Benjamin Y. H. Liu, Jae-Keun Lee, Haskelle Mullins & Susan G. Danisch (1993) Respirator Leak Detection by Ultrafine Aerosols: A Predictive Model and Experimental Study, Aerosol Science and Technology, 19:1, 15- 26, DOI: 10.1080/02786829308959617
[xxviii] WHO Natural ventilation for infection control in health-care settings (https://www.who.int/water_sanitation_health/publications/natural_ventilation/en/) eds: World Health Organization (2009)
[xxix] Hocking M (1998) Indoor air quality: recommendations relevant to aircraft passenger cabins. American Industrial Hygiene Association Journal 59, 446-454
[xxx] Hocking M (2001) Air Quality of Aircraft Passenger Cabins: Ventilation Trends and Potential. Oxford: Aviation Health Institute, 32 pages.
[xxxi] Hocking M (2002) Trends in Cabin Air Quality of Commercial Aircraft: Industry and Passenger Perspectives. Reviews on Environmental Health 17, 1-49.
[xxxii] Institute of Environmental Sciences and Technology. 2016. HEPA and ULPA Filters (IEST-RP-CC001.6). Institute of Environmental Sciences and Technology, Schaumburg, IL.
[xxxiii] Goldsmith CS, Tatti KM, Ksiazek TG, et al. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10(2):320-326. doi:10.3201/eid1002.030913
[xxxiv] Murphy FA, Kingsbury DW. Virus taxonomy. In: Fields BN, Knipe DM, editors. 2nd ed. Raven Press; New York: 1991. pp. 9–35. (Fundamental virology.).
[xxxv] Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557-560. doi:10.1038/s41586-020-2271-3
[xxxvi] Knowles H. 3 July 2019. Mold infections leave one dead and force closure of operating rooms at children’s hospital. Washington Post, Washington, DC. https://www.washingtonpost.com/health/2019/07/03/mold-infections-leave-one-dead-force-closure-operating-rooms-childrens-hospital/?noredirect=on&utm_term=.e14b492b1013
[xxxvii] Nishiura H, Oshitani H, Kobayashi T, et al. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv 2020:2020.02.28.20029272. doi: 10.1101/2020.02.28.2002927
[xxxviii] Kim, S.W., Ramakrishnan, M.A., Raynor, P.C. et al. Effects of humidity and other factors on the generation and sampling of a coronavirus aerosol. Aerobiologia 23, 239–248 (2007). https://doi.org/10.1007/s10453-007-9068-9
[xxxix] Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76(9):2712-2717. doi:10.1128/AEM.02291-09
[xl] Chan KH, Peiris JS, Lam SY, Poon LL, Yuen KY, Seto WH. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv Virol. 2011;2011:734690. doi:10.1155/2011/734690
[xli] Kim SW, Ramakrishnan MA, Raynor PC, Goyal SM. Effects of humidity and other factors on the generation and sampling of a coronavirus aerosol. Aerobiologia (Bologna). 2007;23(4):239-248. doi:10.1007/s10453-007-9068-9
[xlii] BioSpace. 11 February 2020. Condair study shows indoor humidification can reduce the transmission and risk of infection from coronavirus. BioSpace, Urbandale, IA.
[xliii] Noti JD, Blachere FM, McMillen CM, et al. High humidity leads to loss of infectious influenza virus from simulated coughs. PLoS One. 2013;8(2):e57485. doi:10.1371/journal.pone.0057485
[xliv] Kim SW, Ramakrishnan MA, Raynor PC, Goyal SM. Effects of humidity and other factors on the generation and sampling of a coronavirus aerosol. Aerobiologia (Bologna). 2007;23(4):239-248. doi:10.1007/s10453-007-9068-9
[xlv] Marr LC, Tang JW, Van Mullekom J, Lakdawala SS. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface. 2019;16(150):20180298. doi:10.1098/rsif.2018.0298
[xlvi] Xie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17(3):211-225. doi:10.1111/j.1600-0668.2007.00469.x
[xlvii] Kudo E, Song E, Yockey LJ, et al. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc Natl Acad Sci U S A. 2019;116(22):10905-10910. doi:10.1073/pnas.1902840116
[xlviii] Marr LC, Tang JW, Van Mullekom J, Lakdawala SS. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface. 2019;16(150):20180298. doi:10.1098/rsif.2018.0298
[xlix] Kudo E, Song E, Yockey LJ, et al. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc Natl Acad Sci U S A. 2019;116(22):10905-10910. doi:10.1073/pnas.1902840116
[l] Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol. 2002;122(2):183-191. doi:10.1080/00016480252814207
[li] Salah B, Dinh Xuan AT, Fouilladieu JL, Lockhart A, Regnard J. Nasal mucociliary transport in healthy subjects is slower when breathing dry air. Eur Respir J. 1988;1(9):852-855.
[lii] Humphreys S, Deyermond R, Bali I, Stevenson M, Fee JP. The effect of high altitude commercial air travel on oxygen saturation. Anaesthesia. 2005 May;60(5):458-60. doi: 10.1111/j.1365-2044.2005.04124.x. PMID: 15819766.
[liii] Beder A, Büyükkoçak Ü, Sabuncuoǧlu H, Keskil ZA, Keskil S. Preliminary report on surgical mask induced deoxygenation during major surgery. Neurocirugia. 2008;19(2):121-126. doi:10.1016/S1130-1473(08)70235-5
[liv] Overview P. Does the with the respiratory system by inducing oxidative stress and blood oxygen imbalance prolonged use of face-masks by HCWs. Published online 2021. Accessed: 22nd March, 2021: <https://hselibrary.ie/does-the-prolonged-use-of-face-masks-by-hcws-interfere-with-the-respiratory-system-by-inducing-oxidative-stress-and-blood-oxygen-carbon-dioxide-imbalance/>
[lv] Executive S. A health risk assessment of working in hypoxic atmospheres Prepared by the Health and Safety Executive RR1137. Accessed: 22nd March, 2021: <https://www.hse.gov.uk/research/rrhtm/rr1137.htm>